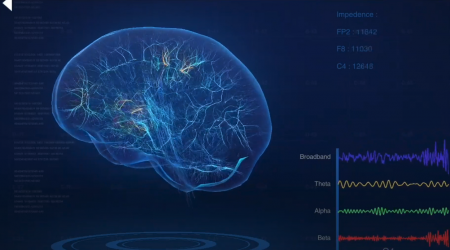
Exsurgo has begun a potentially ground-breaking UK-based clinical trial to determine the effectiveness of Axon, a user-friendly neurofeedback product that treats the primary and secondary symptoms of chronic pain without the use of pharmaceuticals.
Exsurgo is a team of multi-disciplinary professionals in New Zealand and the UK who engineer products that ease human suffering and enhance human potential.
The trial, which began on 8 June 2020, will be a mixed methods proof of concept study to test the safety, efficacy, reliability and validity of Axon.
Richard Little, Exsurgo CEO says, “We’ve taken the existing and clinically accepted EEG technology and miniaturised it into a consumer priced, easily usable, intelligent mobile device to bring mindpower to the people.â€
The low-cost headset and tablet-based software application is poised to reframe the way the world manages health and wellbeing.
While wearing the headset, real-time visual feedback of the brain’s electrical activity associated with pain is displayed in a meaningful way in the application. Users then learn to neuro-modulate this activity to decrease their pain by clinically significant amounts.
Twenty trial participants who suffer from various types of chronic pain will use Axon from the comfort of their own home to measure changes in pain intensity alongside associated symptoms (depression, anxiety, sleep, and quality of life).
“At home care is a significant feature of Axon, given the COVID-19 pandemic, as more clinicians look to future-proof service and product offerings via telehealth,†says Mr Little.
The participants will engage in pre-intervention quantitative assessments to establish a baseline of their primary and secondary symptoms of chronic pain and will then be instructed on how to self-administer the neurofeedback training at home. Training will span four to six sessions per week over an eight-week period.
After the eight weeks, quantitative assessments and analysis will take place to measure the changes to chronic pain and the experience of the neurofeedback training. Participant feedback on how the protocol, equipment and research could be improved will be recorded to inform further research.
Participants will then take part in online follow-up assessments at one, three, and six months after the intervention. Any adjustments to pain medication will also be recorded over this period.
Trial results are estimated to be available by 28 February 2021 with a view to undertaking a larger subsequent multisite trial.
Approximately 13 per cent of people in the UK suffer from moderate-to-severe chronic pain, in part contributing to the global opioid crisis. Axon and the use of EEG neurofeedback as an alternative to pharmacological treatment is primed for success.
The projected benefits of Axon go beyond individual chronic pain management. Dr Nick Birch, a UK-based Orthopaedic Consultant conducted a cost-benefit analysis of Axon and found that “at the greatest saving level (US $1,512 per patient per year), the deployment of Axon has the potential to save the UK healthcare economy in the region of US$7,975m per yearâ€.
More information on the trial can be found here.